TRiAD Study and Consortium
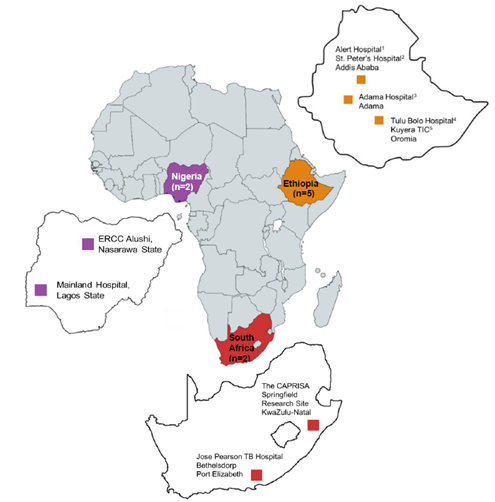
TRiAD is the acronym of a new project “Triage Test for All Oral DR-TB Regimen) funded by the European & Developing Countries Clinical Trials Partnership (EDCTP). It is a multi-center, multi-country study assessing the effectiveness, feasibility, acceptability, and cost-effectiveness of implementing a powerful diagnostic technique the Xpert MTB/RIF (Xpert XDR, Cepheid) for rapid selection of the most appropriate short, all-oral drug-resistant tuberculosis (DR-TB) treatment. An additional aim of the study is to implement the St Andrews-developed quantitative test the Tuberculosis-Molecular Bacterial Load Assay (TB-MBLA) to assess the effectiveness of therapy in real-time. TRiAD is to be launched in 2022 and will recruit patients at 9 sites in 3 countries, Ethiopia (n=5), Nigeria (n=2), and South Africa (n=2) running the study for 48 months.
The St Andrews team will provide Medical Monitoring as well as support the implementation of TB-MBLA led by the National Institute for Medical Research (NIMR) Mbeya Medical Research Centre. To prepare the sites for the trial, Dr Wilber Sabiiti (University of St Andrews) and Dr Bariki Mtafya (NIMR-Mbeya, Tanzania) delivered training at the Institute of Human Virology in Nigeria in November 2021. The training has provided laboratory scientists the skill required to test and quantify MTB bacterial load using the new PCR technique tool (TB-MBLA). TB-MBLA will be used as an adjunct to provide bacillary load monitoring over the course of treatment to assess real-time treatment response and hopefully provide better treatment outcomes for the patient.