REMoxTB
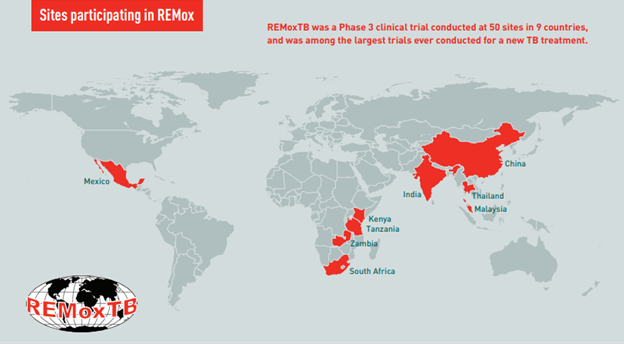
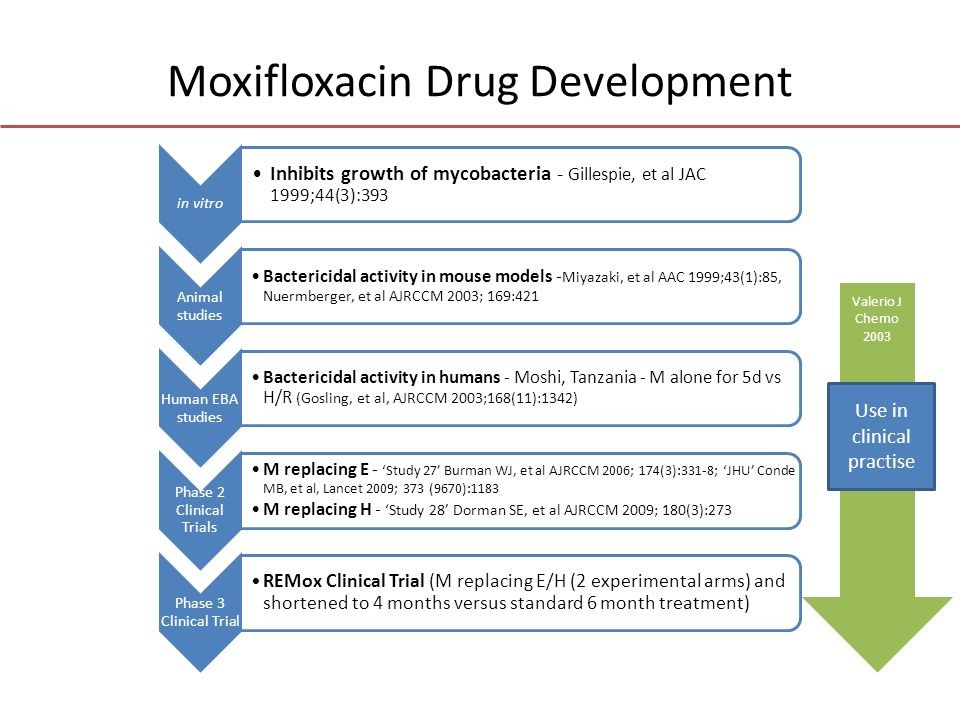
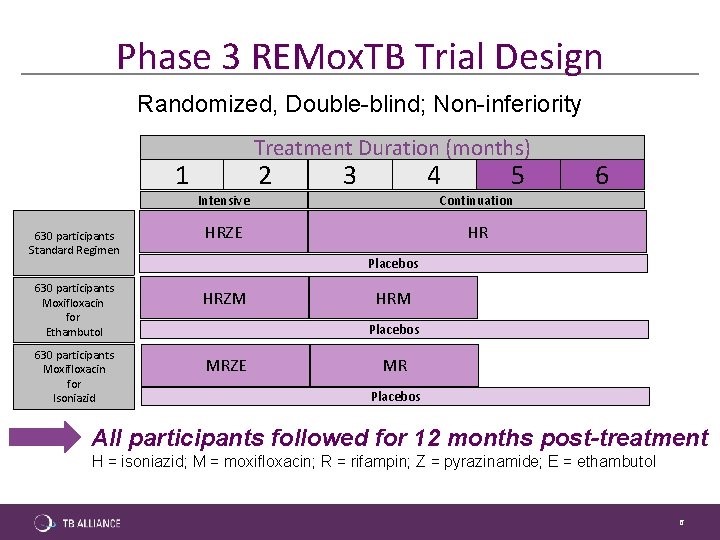
REMoxTB was a global Phase 3 clinical trial conducted at nearly 50 sites. The trial tested whether substituting moxifloxacin for a single drug in the standard TB therapy could reduce treatment of drug-sensitive disease from six months to four.
What was the study about?
REMoxTB was conducted at 50 sites around the world by TB Alliance, Bayer Healthcare AG, University College London, and the Medical Research Council. It was the first Phase 3 TB drug trial conducted and completed in accordance with the most stringent modern regulatory standards, and it helped pave the way for subsequent clinical research and influence regulatory standards in the field. The trial was also conducted with significant involvement from local communities through community advisory structures promoted by TB Alliance to give communities a voice in the research process.
What did the study show?
As reported in the New England Journal of Medicine, the REMoxTB Phase 3 trial showed that the two regimens tested in the trial were found to be safe, but did not shorten the time it takes to treat drug-sensitive TB from six to four months.
The study showed that the experimental regimens were more bactericidal in the earlier stages, however, patients were more likely to relapse in the year following therapy. More importantly, the study showed that moxifloxacin-containing treatments were safe and appeared to be less toxic to the liver, a common problem in tuberculosis therapy, than standard therapy.
Due to the high relapse rate post-therapy, there was insufficient effect to permit regimen shortening by two months.