Simplifying treatment for drug-sensitive and -resistant tuberculosis
Therapy for tuberculosis is lengthy and expensive. The long duration of treatment and the high burden of pills to be taken daily makes it difficult for some patients to complete therapy. A multidrug regimen of four antibiotics for 6 months is prescribed for drug-sensitive tuberculosis, while more complicated drug-resistant strains require therapy of 9 to 30 months to be eliminated with only a 50% success rate. The drugs used to treat drug-resistant TB can be both orally admitted or injectable and are known to have serious side effects including deafness and blindness. Besides, the difficulties experienced by patients undergoing treatment for TB often lead to non-adherence to therapy which increases the risk of drug resistance and negative treatment outcomes such as relapse.
Novel compounds with promising treatment shortening activity
Bedaquiline (B) and pretomanid (Pa) are novel drugs that recently have been undergoing evaluation in pre-clinical and early phase clinical studies and have shown activity against M. tuberculosis bacteria. Bedaquiline is an FDA and EMA-approved drug already in use for drug-resistant tuberculosis. It has been shown to be active against dormant organisms with promising sterilising activity. Pretomanid is a new chemical entity in clinical development which possesses significant anti-tuberculosis activity and a unique mechanism of action. Most recently, pretomanid has also been approved for use in the treatment of drug resistant tuberculosis.
Supported by an extensive portfolio of in vitro, animal, pre-clinical and clinical studies the SimpliciTB study is testing a novel combination of these drugs complemented by moxifloxacin (M) and pyrazinamide (Z) for 4 and 6 months in drug-sensitive and resistant TB, respectively.
Phase 3 clinical evaluation of the BPaMZ regimen
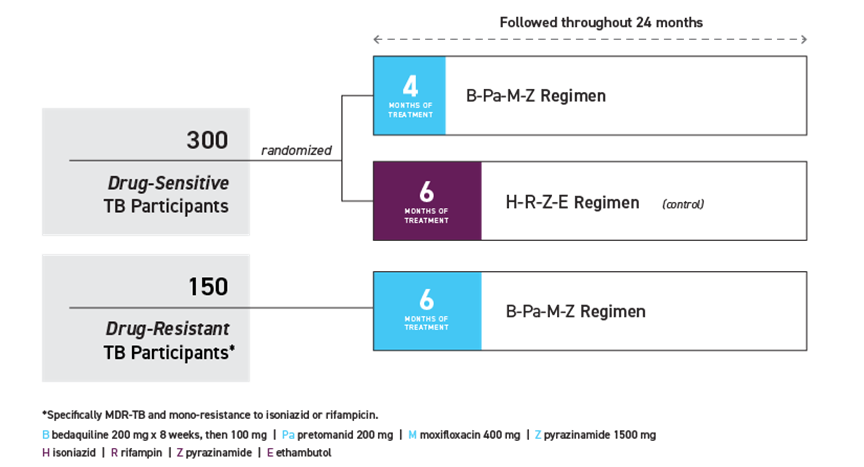
SimpliciTB is a multi-centre phase 3 clinical trial evaluating a new shorter drug regimen (all-oral BPaMZ) in drug susceptible and resistant pulmonary tuberculosis across 4 continents led by TB Alliance.
The first patients have been enrolled at the National Centre for Tuberculosis and Lung Disease in Tbilisi, Georgia. SimpliciTB is expected to enrol 450 people with TB, including up to 150 with MDR-TB* across at least 26 centres in ten countries in Africa, Asia, Europe, and Latin America.
Mel Spigelman, president, and CEO of TB Alliance, said: “As resistance to current TB treatments continues to grow, we need to introduce all-oral drug regimens that can treat every person with TB in six months or less, regardless of their resistance profile.
Medical Monitoring in the SimpliciTB study
The Medical Monitoring team from the University of St Andrews and South Africa assist site investigators to apply appropriate criteria for patient recruitment. Monitors are also available to discuss individual cases where the interpretation of the protocol is unclear.
Medical monitors
- support the development of clinical aspects of the trial’s protocol
- establish safety monitoring forms and lines of reporting
- ensure participant safety and assist in the safe management of adverse events.
- support the sites in reporting adverse events
- support definition of patient final outcome
- maintain regular contact with sites to resolve issues as they arise
This project is part of the EDCTP2 programme supported by the European Union.
Sponsored by: