Tuberculosis Clinical Trials
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain evidence for a process considered effective as a medical treatment. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. TB clinical trials can generally fall into two category: interventional (drug) trials and diagnostic trials.
Interventional (drug) Trials
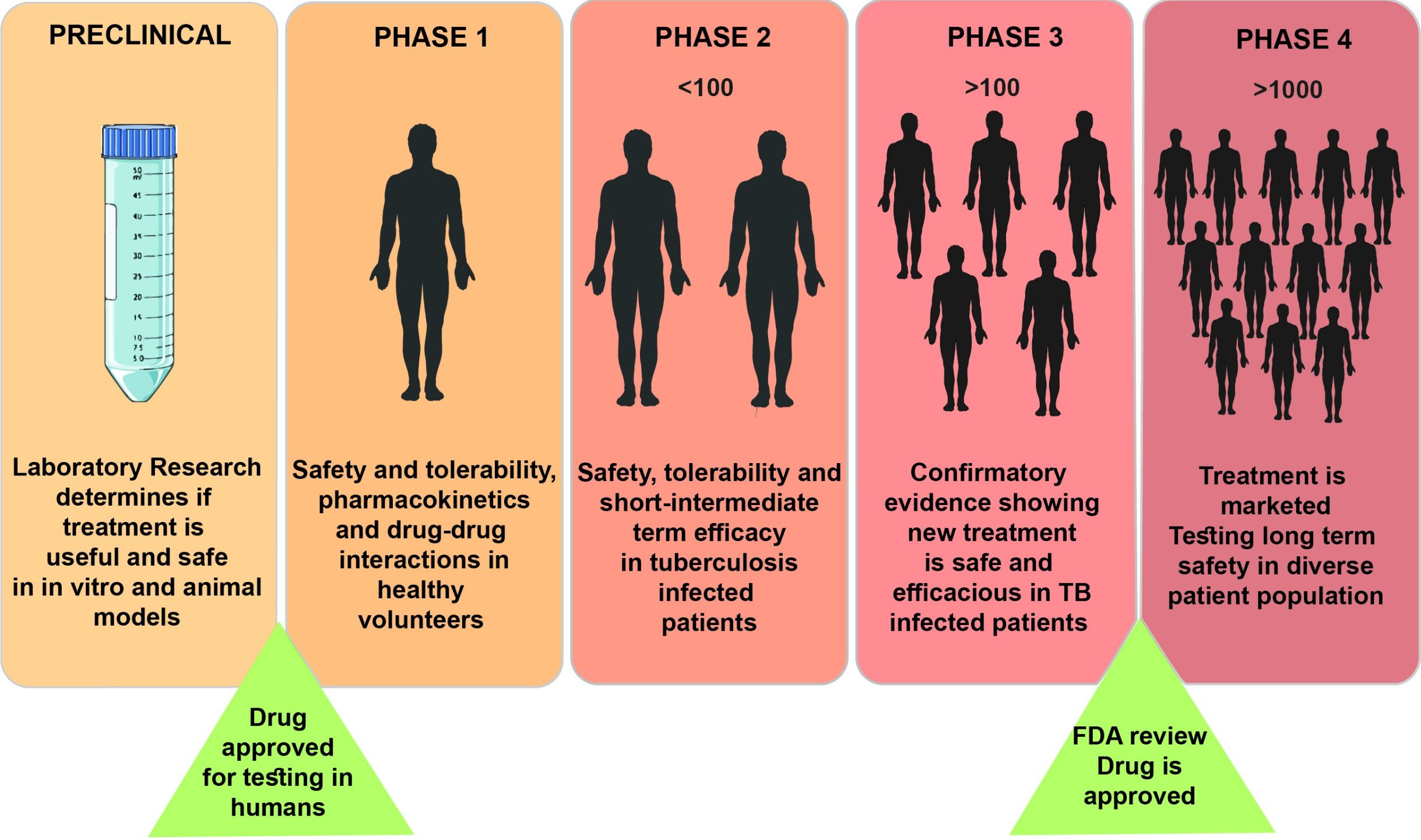
Drug development begins in preclinical studies where researchers find out about the safety and biological efficacy of a drug candidate before testing it in human population. Pre-clinical studies are usually performed in research laboratories by scientists using models that mocks the environment the drug is intended to act on its target. Animal models are also used in the final stage of preclinical research.
The clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. In interventional trials a new pharmaceutical intervention is tested – such as a new antibiotic or a new drug regimen with prospective benefits over the currently used therapy. The most common interventional trials in TB are those testing new combination of TB drugs that can shorten the duration of therapy from 6 months to an aimed 4 months or less. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective.
Phases of an interventional clinical trial
Diagnostic trials
Diagnostic trials have a crucial role in breaking the trajectory of the TB epidemic reaching the global targets of a world free of tuberculosis. One of the three pillars of the WHO established End TB strategy is supporting and prioritising the research of new diagnostics. There is a high need for better diagnostic tools in TB which can substitute the current laboratory methods which are labour intensive and time consuming. Our team has a profound expertise in developing and evaluating new tools for diagnosing TB, which is frequently incorporated into the Ph.D. projects of our enthusiastic researchers. Over the years we have invented new tools for easier and faster detection of M. tuberculosis which methods can be implemented in clinical trials and everyday clinical practice.