Shorter Treatments by Advancing Novel Drugs
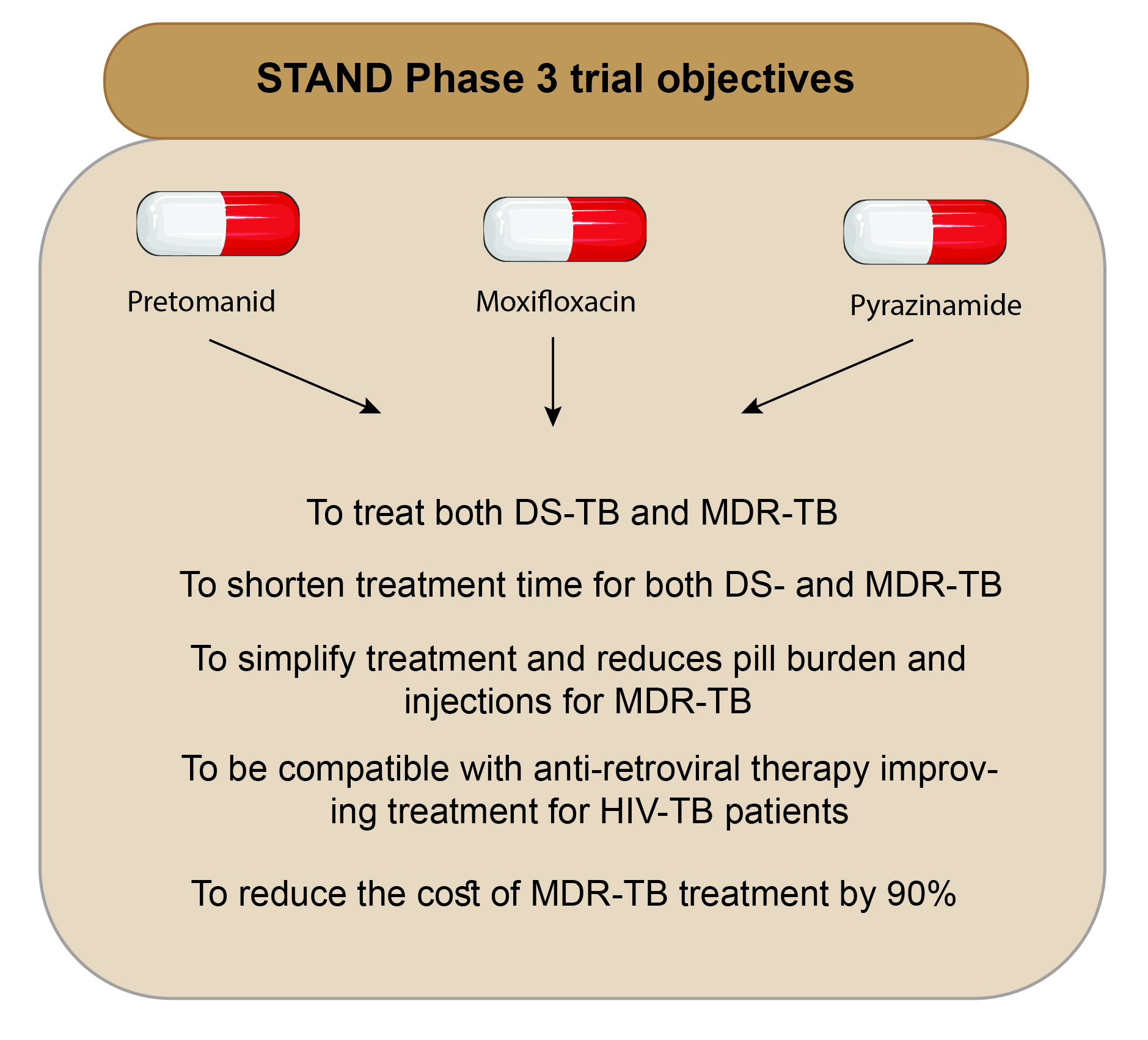
The Shortening Treatment by Advancing Novel Drugs (STAND) trial is a phase 3 open-label partially randomised clinical trial investigating the treatment shortening effect of a new regimen PaMZ. The study was funded by TB Alliance and tested the safety, tolerability, and efficacy of regimen pretomanid (PA-824), moxifloxacin (M), and pyrazinamide (Z) based combinational TB therapy.
PaMZ was the first drug regimen designed to treat both drugs-susceptible and -resistant TB and be compatible with anti-retroviral therapy.
Nevertheless, all of the participants of the study were followed as per the protocol even after the enrolment ceased. Patients completed treatment and a six month follow up as well as additional monitoring for two years (Source TB Alliance)
STAND was paused in late 2015 by which time 284 patients had been enrolled. The study was eventually not re-started due to another trial testing BPaMZ regimen, which appeared to be more promising than the PaMZ regimen.