Archived News
SimpliciTB Strategic African network meeting – sustaining research capacity, Dar es Salaam, Tanzania 28th-30th September 2022
Our collaborators from the Global South assembled from NIMR-Mbeya, KCRI, TASK IHI, KIDH, KCRI, NIMR-Mbeya, NIMR-Mwanza, TASK, CERMEL, UCT, Wits Health, AURUM, and INSA gathered in Dar es Salaam on the 28th – 30th September.
Speaking via video link, Professor Stephen Gillespie opened the conference “Once upon a time in a country far far away: TB research in the 1990s”. This presented an overview of tuberculosis research in 1990 and how the collaboration had participated in a very significant change in research capacity through global collaboration in (PanACEA, EDCTP, TB Alliance link). Through a summary of our collaborative research: clinical phase 1-3 trials, and monitoring TB treatment response with molecular techniques TB-MBLA, he outlined how the research dynamic has changed together with some observations for the conference and the future.
One of the SimpliciTB Capacity Development aims is to strengthen research capacity to include the development of research leadership in sub-Saharan Africa. The SimpliciTB network has organized research capacity leadership into 4 workstreams addressing the training of various cadres involved in clinical trials, biomarkers, clinical trials, operational research, and community engagement measurement and evaluation. Implementation of workstreams activities prepares the Sub-Saharan Africa investigators to take leadership roles in the near future.
Dr Stella Mpagama was endorsed at the meeting as the African lead to coordinate colleagues to focus on simplifying TB treatment and apply regulatory phase III/IV trials to improve the capacity of sites. Validating TB diagnostics and drugs and future investment in infrastructure to support TB research work. A particular focus will be to invest in recruiting and developing young investigators and providing a supportive network for administrators, finance officers, and grant managers.
TB-MBLA Quarterly Newsletters are back again!
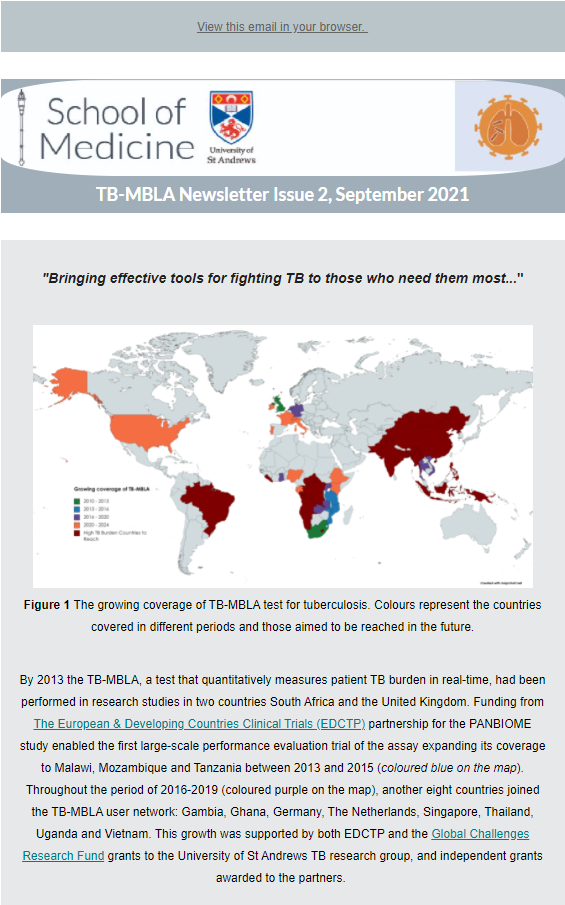
One of the focuses of the many clinical studies we conduct and participate in is the successful implementation of novel biomarkers in the clinical trial workflow to evaluate their performance and feasibility compared to standard microbiology practice. TB-MBLA has been a promising molecular tool that simplifies TB treatment monitoring by significantly reducing the turn- around time of quantitative bacterial load results. In doing so, patients’ responses to therapy can be followed real-time and with higher precision. The TB-MBLA research & implementation team led by Dr Wilber Sabiiti at the University of St Andrews is proud to have established a TB-MBLA User Group that consists of both research and industrial partners using and testing the performance of the assay across the world. With the purpose of maintaining the connection and communication with the members of the group, the idea of a TB-MBLA newsletter had arisen in previous years. In 2021 the newsletter was published on two occasions in September and in December.
In the September issue, we featured research news from Uganda, Tanzania, and St Andrews. Emmanuel Musisi is doing a joint PhD project between the University of St Andrews and Makarere University where he is currently conducting a field study evaluating the diagnostic accuracy of TB-MBLA as a diagnostic test. His findings are promising so far showing high accuracy of TB-MBLA in diagnosing pulmonary tuberculosis from sputum samples. Meanwhile, scientists at the National Institute for Medical Research (NIMR) in Mbeya are conducting an implementation study of TB-MBLA in routine healthcare settings. The intermittent results of the study have been showcased in the newsletter and were presented at the 52nd Union World Conference on Lung Health. Finally, the TB research team at the University of St Andrews is developing molecular tools for the detection of key bacterial species associated with chronic obstructive pulmonary disease using the TB-MBLA principle. More information on this project and news on the industrial development of the assay can be read in the newsletter.
In the December issue, we reported on an extensive laboratory training that took place in the Institute of Human Virology in Nigeria on 22nd -26th November 2021. During the course, trainees learned how to perform TB-MBLA in their laboratory which has successfully prepared them for the soon-to-be-launched TRiAD study. In this trial, TB-MBLA will be used and evaluated to monitor patients’ bacterial load in response to short, all-oral drug-resistant TB therapy. We were also delighted to announce that TB-MBLA has been listed in the TB Diagnostic Pipeline for 2021 by the Treatment Action Group (TAG). More about our December news can be read in the newsletter.